#chemistry
Rockin’ out
IBM researchers have made a new breakthrough in the study of nanoparticles. Because of nanoparticles’ naturally erratic movement and small size (about 1,000 times smaller than the diameter of a human hair), they’re notoriously difficult to separate and control – and study. IBM researchers created a motor device for sorting, separating and moving nanoparticles without the need for water, which could eventually lead to lab-on-a-chip applications. Using a “rocking” motion and oscillating electrical field across the nano-landscape, the device can more precisely separate nanoparticle populations; a model suggests that it can separate nanoparticles ranging from 5 nanometers (nm) to 100 nm. This technology has major implications in the fields of material science, environmental science and biochemistry, such as potentially helping eradicate drinking water pollutants.
Learn more about rocking Brownian motors ->
Post link
That was not a good date
Was he nice? Yes
Was he talkative? Yes
Did he offer to pay and refuse me helping to pay? Yes
HOWEVER
Did we have chemistry? No…
Did I find him attractive? No…
Do I want to see him again? No…
I’m sure people will judge me for this and say, ‘you can’t have everything, he was a gentleman and nice to you but not good enough you’ll never be happy etc etc’.
Let me say this;
Just because a guy pays for you (which he doesn’t have to) and is pleasant to talk to and nice, does not mean you are entitled to see them again
yes, he was a nice person, yes, i’d see him again on a friendly basis
But I am not going to subject myself to dating someone and messing them around when I know full well I didn’t find them sexually attractive or feel as though we had any sparks or chemistry
Life is too short to be unhappy for the sake of others
- Becky
Why is breath sometimes cold and sometimes warm? (”hoo vs. haa”)
Hold your hand about a half foot (15 cm) from your mouth and open your mouth wide and blow air like you are fogging up a mirror. (”haa”) Your breath should feel warm. Now purse your lips and blow out. (”hoo”) Your breath should now feel cold. If you bring your hand closer to your mouth by about a centimeter away and blow out through pursed lips then your breath should feel warm again.
Why is this?
The air from your exhale is generally warmer than the ambient air outside your mouth. When your lips are pursed then the air is moving at higher speeds than with your mouth open. At these higher speeds the air from the exhale drags along the cooler still air due to friction. The breath air thus is doing work on this cooler so it also looses energy resulting in lower temperature. So overall the added cooler air and decreased temperature makes your breath feel cooler.


At distances closer to your mouth the warm breath air has yet to lose enough energy or drag along any cooler air so the breath still feels warm. With your mouth open the air is slower and takes up a larger volume so the majority of the air that reaches your hand is warm.

materialsscienceandengineering:
Scientists break record for highest-temperature superconductor: Experiment produces new material that can conduct electricity perfectly
University of Chicago scientists are part of an international research team that has discovered superconductivity–the ability to conduct electricity perfectly–at the highest temperatures ever recorded.
[…]
Using advanced technology at UChicago-affiliated Argonne National Laboratory, the team studied a class of materials in which they observed superconductivity at temperatures of about minus-23 degrees Celsius (minus-9 degrees Fahrenheit)–a jump of about 50 degrees compared to the previous confirmed record.
Though the superconductivity happened under extremely high pressure, the result still represents a big step toward creating superconductivity at room temperature–the ultimate goal for scientists to be able to use this phenomenon for advanced technologies. The results were published May 23 in the journal Nature; Vitali Prakapenka, a research professor at the University of Chicago, and Eran Greenberg, a postdoctoral scholar at the University of Chicago, are co-authors of the research.
Post link
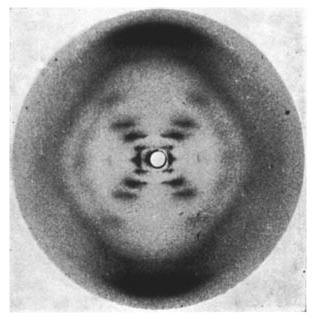
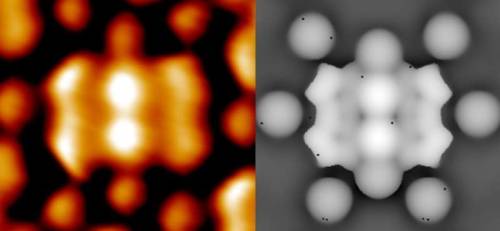
Many of you may recognize this photo of the x-ray diffraction pattern of DNA found by Rosalind Franklin and her PhD student, Raymond Gosling. But, you may wonder how one could figure out from this image that DNA is structured as a double helix and even how x-ray crystallography works.
X-Ray Crystallography
X-ray crystallography is a method of determining the positions and arrangements of atoms in a crystal. Crystals are usually defined to be a highly ordered and repeating microscopic structure of a solid rather than the macroscopic crystals we know like quartz which actually tend to be “polycrystals” because at a microscopic level they do have the highly ordered structure required. Ice is also a polycrystal composed of many smaller ice crystals.
1.) X-ray beams are shot at the crystals
The x-rays interact with electrons of the atoms. This interaction or collision is typically modeled by Thomson scattering where the energy and thus frequency of the x-rays do not change after diffraction. This is similar to light going through a diffraction grating.
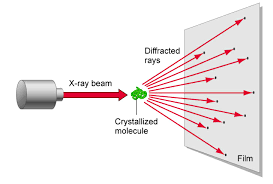
2.) Beam is diffracted
The x-rays are diffracted based on the crystal lattice structure of the substance. This is dependent on the characteristics of the bonds between atoms like the bond angles and bond lengths. Also the spacing between molecules also determines the diffraction.

3.) Diffraction pattern
The diffracted x-rays are light waves so they interfere both constructively and destructively. The resulting intensities of the x-rays are recorded on a screen behind the sample to create a diffraction pattern. The sample is rotated to take more data. After sufficient data is taken a model for the crystal structure for the sample can be developed. With a diffraction pattern an electron density map can be made which depicts the location and size of electron clouds in the substance.

Above is an example of an electron density map.
Fun-o-fact #1
If two pieces of the same type of metal touch in space, they will bond and be permanently stuck together.
materialsscienceandengineering:
Giant lasers crystallize water with shockwaves, revealing the atomic structure of superionic ice
Scientists from Lawrence Livermore National Laboratory (LLNL) used giant lasers to flash-freeze water into its exotic superionic phase and record X-ray diffraction patterns to identify its atomic structure for the very first time—all in just a few billionths of a second. The findings are reported today in Nature.
In 1988, scientists first predicted that waterwould transition to an exotic state of matter characterized by the coexistence of a solid lattice of oxygen and liquid-like hydrogen—superionic ice—when subjected to the extreme pressures and temperatures that exist in the interior of water-rich giant planets like Uranus and Neptune. These predictions remained in place until 2018, when a team led by scientists from LLNL presented the first experimental evidence for this strange state of water.
Now, the LLNL scientists describe new results. Using laser-driven shockwaves and in-situ X-ray diffraction, they observe the nucleation of a crystalline lattice of oxygen in a few billionths of a second, revealing for the first time the microscopic structure of superionic ice.
Post link
Today’s FYFD video tells a story I’ve wanted to share for a couple of years now. It’s about the life and work of Agnes Pockels, a woman born in the mid-nineteenth century who, despite a lack of formal scientific training, made major contributions to the understanding of surface tension and to the experimental apparatuses and methodologies used in surface chemistry in general. She accomplished all of this not in a scientific lab, but from her kitchen.
Pockels’ story is one of curiosity, determination, and meticulous scientific inquiry. Chances are that you’ve never heard of her, but you really should. Check out the full video below to learn more! (Image and video credit: N. Sharp)
Post link
Researchers uncover basics of common industrial catalytic processes
Catalysts are used in a wide variety of industrial processes around the world in everything from the production of medicines, fertilizers, plastics, and other household products to the processing of fossil fuels.
They speed up chemical reactions with the aim of minimizing energy usage. But while they are critically important, catalysts have often been developed through trial and error or tradition rather than through scientific principles.
Using a combination of microscopy and spectroscopy to get real-world imagery as well as sophisticated theoretical calculations, Washington State University researchers collaborated with Prof. Junfa Zhu from the University of Science and Technology of China to unravel an underlying mechanism of a catalytic reaction at the atomic level.
The work, published in the journal of the American Chemical Society, JACS, improves fundamental understanding of reactions that could someday lead to more efficient industrial processes.
Post link
From left to right: Nickel (II) sulfate, Potassium alum, Barium chloride, Copper (II) sulfate, Cobalt (II) chloride, Sodium Chloride and Potassium permangante.
Post link





All of these samples were collected at Hogen Camp Mine, Harriman State Park, NY. The first image is a reflected light image of the ore vein. The ore vein formed as a result of dextral shear which ultimately created large fractures. Shortly after this, hydrothemal alteraltion occured of the metavolcanic gneiss in the region (image 2 and 3). The metavolcanic gneiss is rich in iron. Due to this, the highly acidic metamorphic fluids began to precipitate in the fractures. The process yeilded magnetite, clinopyroxene, and less common biotite within the fractures occuring at Hogen Camp Mine. The clinopyroxene and biotite are highly rich in iron.
Image 3 and 4 is the local pink pegmatites that occured in the region around 923 Ma. The pegmatitic dikes formed post-Ottawan orogeny. Composition includes: alkali feldspar with minor constituents of clinopyroxene and quartz.

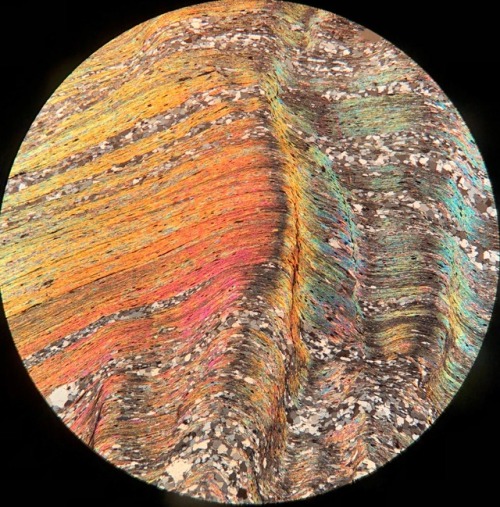
This rock is a quartzofeldspathic gneiss from Surebridge Mine in Harriman State Park, NY. What’s so cool about this is you can see the hydrothermal process which alters biotite to chlorite. The large brown grain being biotite, and the purple/blue/green in the center being chlorite. (10x XPL)
Working on polishing my samples for reflective light microscopy. This sample is a pyroxenite from Hogen Camp Mine in the Hudson Highlands region of New York. The area was once a lead producer of iron ore and magnetite for the east coast.
Post link
Alkali Feldspar crystals are large in this thin section. The alkali feldspar have subhedral crystals. The quartzy matrix in some areas is intergrown at the edge of the alkali feldspar crystal faces. The muscovite crystallized in the interstitial space and have anhedral crystal faces. To differentiate between the muscovite and the biotite pleochroism comes into effect. The biotite is darker amber under PPL and muscovite is tan/light brown. Both are pleochroic under PPL.
Post link
This rock is predominantly composed of quartz, alkali feldspar, and plagioclase. The alkali feldspar grains are large and take up a decent amount of the slide. The perthite is mainly one grain that is large and is about 11mm. The plagioclase has
polysynthetic twinning.The muscovite is pleochroic under PPL and has clinopyroxene embayed in the core. Clinopyroxene is present because the melt reacted with the clinopyroxene and create muscovite. Leucite is also present in a minimal quantity. The leucite is embayed in the alkali feldspar. This happened when the alkali feldspar started to crystalize. The silica content was too great and the leucite reacted with the melt to form alkali feldspar. Due to the composition the rock would just be classified as a granite.
(leucite in 2nd pic)
Post link
Composition includes: biotite, muscovite, chlorite, feldspars, oxides, and quartz. Feldspar poikiloblasts with inclusions of quartz and oxides are prominent throughout much of the sample. Most poikiloblasts occurred synkinematic to the 1st deformation event (D1) and rotation occurred. D1 had a direct effect on the foliation (S1) of minerals that already crystallized. D2 led to crystallization of quartz which is seen in quartz ribbons and pressure fringes throughout the sample.
Post link
This thin section shows an amygdule in a sample of amygdaloid basalt. The rock formed due to an eruption of gaseous, low viscous magma which resulted in vesicles throughout much of the rock. The rock then underwent hydrothermal alteration and low temperature alteration minerals formed in the once vesicles, forming amygdules. The amygdules are composed of quartz, celadonite (a type of mica) and epidote.
Post link
Websterite in thin section
Clinopyroxene and orthopyroxene with few anhedral plagioclase crystals in interstitial space. Opaques (oxides) also present.
Post link